Title: Solvation Effect-Determined Mechanisms of Cation Exchange Reactions for Efficient Multicomponent Nanocatalysts
Authors: Shangheng Liu, Xiaocan Wang, Wei-Hsiang Huang, Qiugen Zhang, Jiajia Han, Yingtian Zhang, Chih-Wen Pao, Zhiwei Hu, Yong Xu, Xiaoqing Huang*
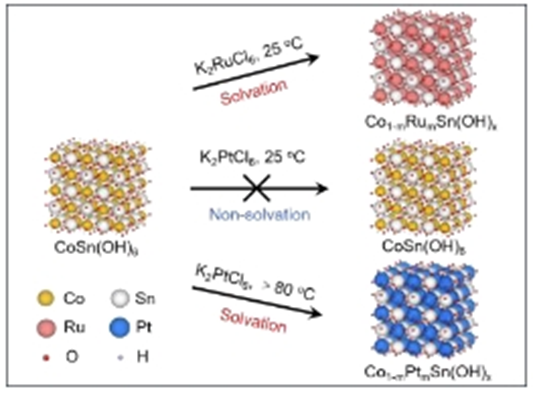
Abstract: Cation exchange (CE) reaction is a classical synthesis method for creating complex structures. A lock of study on intrinsic mechanism limits its understanding and practical application. Using X-ray absorption spectroscopy, we observed that the evolution from Ru−Cl to Ru−O/OH occurs during the CE between K2RuCl6 and CoSn(OH)6 in aqueous solution, while CE between K2PtCl6 and CoSn(OH)6 is inhibited due to the failure of structural evolution from Pt−Cl to Pt−O/OH. Theoretical simulations imply that the interaction between Ru−O and CoSn(OH)6 with Co vacancy (CoVCoSn(OH)6) endows the electron transfer, as a result of strengthened adsorption on CoVCoSn(OH)6. Moreover, this mechanism is validated for CE between K2RuCl6 and ASn(OH)6 (A = Mg, Ca, Mn, Co, Cu, Zn), and CE between K2PdCl6/Na3RhCl6/K2IrCl6 and CoSn(OH)6. Impressively, the Pt-free CoRuSn(OH)x produced via CE displays a mass activity and a power density of 15.0 A mgRu−1 and 11.6 W mgRu−1, respectively, for anion exchange membrane fuel cell (AEMFC) exceeding the values of commercial PtRu/C (11.8 A mgRu+Pt−1 and 9.0 W mgRu+Pt−1). This work, for the first time, reveals the intrinsic mechanism of CE as structural evolution of target ion breaking through the traditional classic etch-adsorption mechanism and will promote fundamental research and practical application in various fields.
Full-Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202418248